 Reading Notes for Chapter 15
Reading Notes for Chapter 15 Reading Notes for Chapter 15
Reading Notes for Chapter 15An organic acid is (usually) defined by the presence of a carboxylic acid group, and has a name that ends in "-oic acid". Carboxylic acids are named in the same way as the other organic molecules we've looked at... starting with the parent alkane (or alkene or alkyne), replace the final "e" with "-oic acid" to name the carboxylic acid. For example, If we have a carboxylic acid whose longest continuous chain is 6 carbons long and it has all single C-C bonds, no branches, and no rings, we'd call it "hexanoic acid" by replacing the final "e" in "hexane" with "oic acid".
The carboxylic acid group is always at the end of a chain (where a methyl group would be in an alkane chain), and the carbon has a double-bonded oxygen and a hydroxyl group attached. The carbon of the carboxylic acid is designated as "1" when numbering the carbon chain. Because the carboxylic acid group is at the end of the chain and is always considered the "1" position of the chain, we do not have to include a numeric designation to specify where the carboxylic acid group is. For example, "hexanoic acid" is a sufficient unambiguous name... we would never have to specify "1-hexanoic acid". If the parent hydrocarbon was hexene instead of hexane, we would need to include a numeric designation to specify where the double bond is. For example, "2-hexenoic acid" or "5-hexenoic acid". Pop Quiz: Why could we have a molecule called "1-hexenoic acid"? Try drawing the structure...
Because many carboxylic acids have been known for centuries, there are many common names that are used regularly. I mention these here because you will encounter them in the real world, but I will typically use systematic IUPAC names for all carboxylic acids.
| IUPAC Name: |
Common Name: |
| methanoic acid | formic acid |
| ethanoic acid | acetic acid (this is one common name that I will likely use because it is so common) |
| propanoic acid | propionic acid |
| butanoic acid | butyric acid |
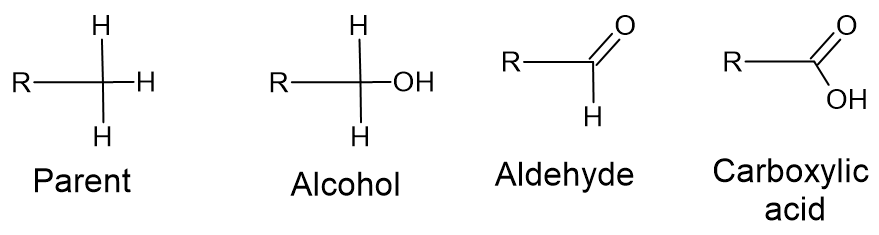
Carboxylic acids can be most readily formed by oxidation of an alcohol to an aldehyde and then to a carboxylic acid. If we start from the parent hydrocarbon, each "step" of the oxidation reaction replaces one C-H bond with a C-O bond.
Similar to other organic structure types we've already looked at, the boiling points of a homologous series of carboxylic acids increases with increasing chain length. Because of the strong hydrogen bond interactions between carboxylic acid groups, the melting points of short chain carboxylic acids do not follow a predictable pattern. Water solubility of carboxylic acids decreases with longer chains.
When a carboxylic acid donates its proton, it becomes a carboxylate ion. Carboxylates are (usually) more soluble in water than their carboxylic acid parent compound.
One of the most important reactions that carboxylic acids undergo is the reaction with an alcohol to form an ester. In an ester, the "H" of the parent carboxylic acid is replaced by some hydrocarbon group that is supplied by the parent alcohol. Esters are named by combining the name of the alcohol parent with the carboxylate form of the name of the carboxylic acid parent. For example, if methanol and butanoic acid react to form an ester, the resulting ester would be called methyl butanoate. Note: In an esterification reaction, the oxygen of the ester comes from the alcohol starting material, not the carboxylic acid starting material.
Esters often have distinct (and distinctly pleasant) smells and are responsible for the fragrance of many flowers, fruits, and other food items. A very small change in either the carboxylate or the alcohol-derived part of the ester can change the detected odor significantly; for example, "pear" vs "banana" for pentyl acetate vs iso-pentyl acetate. In these 2 esters, the only difference is that in pentyl acetate, the 5-carbon chain is attached at the "1" position while in isopentyl acetate the 5-carbon chain is attached at the "2" position. Humans (and many other animals) have evolved to be very sensitive to the smell of different esters because they exist in so many foods.
Esters are polar compounds, but they are not able to hydrogen bond with other esters because the acidic "H" of the carboxylic acid has been replaced with a hydrocarbon "R" group. This makes their melting and boiling points lower than the parent carboxylic acids. {Note: This is somewhat dependent upon the alcohol used to make the ester. If a HUGE alcohol is used, it will affect the melting/boiling points quite a bit.}
Esters can undergo hydrolysis in the presence of water and acid to revert to the original carboxylic acid and alcohol. Hydrolysis is the reverse of the esterification reaction shown above. Esters can also undergo hydrolysis in the presence of base (hydroxide ions) to form a carboxylate salt and alcohol.
It is also possible to form "esters" of some oxoanions such as phosphoric acid and sulfuric acid. These "mineral esters" are especially important in biological systems, especially the esters of phosphoric acid.
The most common class of organic base are the amines, R-NH2. Amines can accept H+ to become alkylammonium ions, just like ammonia can accept H+ to become ammonium ions. Amines often have strong (and often unpleasant) odors, many of which are associated with decay or spoilage. Amines can be thought of an a nitrogen analogue of an alcohol.
The number of carbon chains attached to the nitrogen of an amine is designated by the terms "primary" (1°, 1 carbon attached to nitrogen), "secondary" (2°, 2 carbons attached to nitrogen), and "tertiary" (3°, 3 carbons attached to nitrogen). There are also "quaternary" (4°, 4 carbons attached to nitrogen) amines, but these are charged and cannot accept H+ because there are already 4 bonds to the central nitrogen so these amines usually are considered a separate class of compounds.
Caution!! When comparing amines to alcohols, the designations "primary", "secondary", and "tertiary" have different meanings. Make sure you know what type of molecule and functional group you are talking about when you use these terms!
Amines are named either by simply naming the alkyl group followed by "amine" (for example, "1-butylamine") or by naming the "amino" group the same way we would designate any other substituent (for example, "1-aminobutane"). The second method is more common in molecules that have multiple different types of functional groups.
Amine groups can undergo hydrogen bonding interactions, but "N-H" hydrogen bonds are generally weaker than "O-H" hydrogen bonds, so amines generally have higher melting/boiling points than their parent alkanes, but lower melting/boiling points than similar alcohols or carboxylic acids.
There are many examples of cyclic amines in living systems. These "heterocyclic amines" adopt many of the same cyclic structures as cyclic hydrocarbons with "N" or "N-H" or "N-R" replacing one of the carbons in the cyclic structure. Nitrogen-containing heterocycles found in plants are often classified as "alkaloids" and can have a variety of drug properties ranging from therapeutic to hallucinogenic to poisonous.
The simplest amides simply replace the "-OH" of a carboxylic acid with "-NH2". These compounds are named by replacing the "-oic acid" part of the parent carboxylic acid name with "-amide". Similar to their related carboxylic acids, amides can form relatively strong hydrogen bonds and are very polar, which lead to relatively high melting/boiling points as compared to alcohols, amines, and hydrocarbons of similar size.
Amines and alcohols share some similar chemical properties, including their ability to react with carboxylic acids. When an alcohol reacts with a carboxylic acid, it forms an ester. When an amine reacts with a carboxylic acid, it forms an amide. Amide bonds are incredibly important in industry and biochemistry. Nylon is a class of amide-bonded polymer used in a wide variety of applications. Polyacrylamide is polymer with amide pendant groups that strongly influence its physical properties.
Amides can undergo hydrolysis similar to esters.